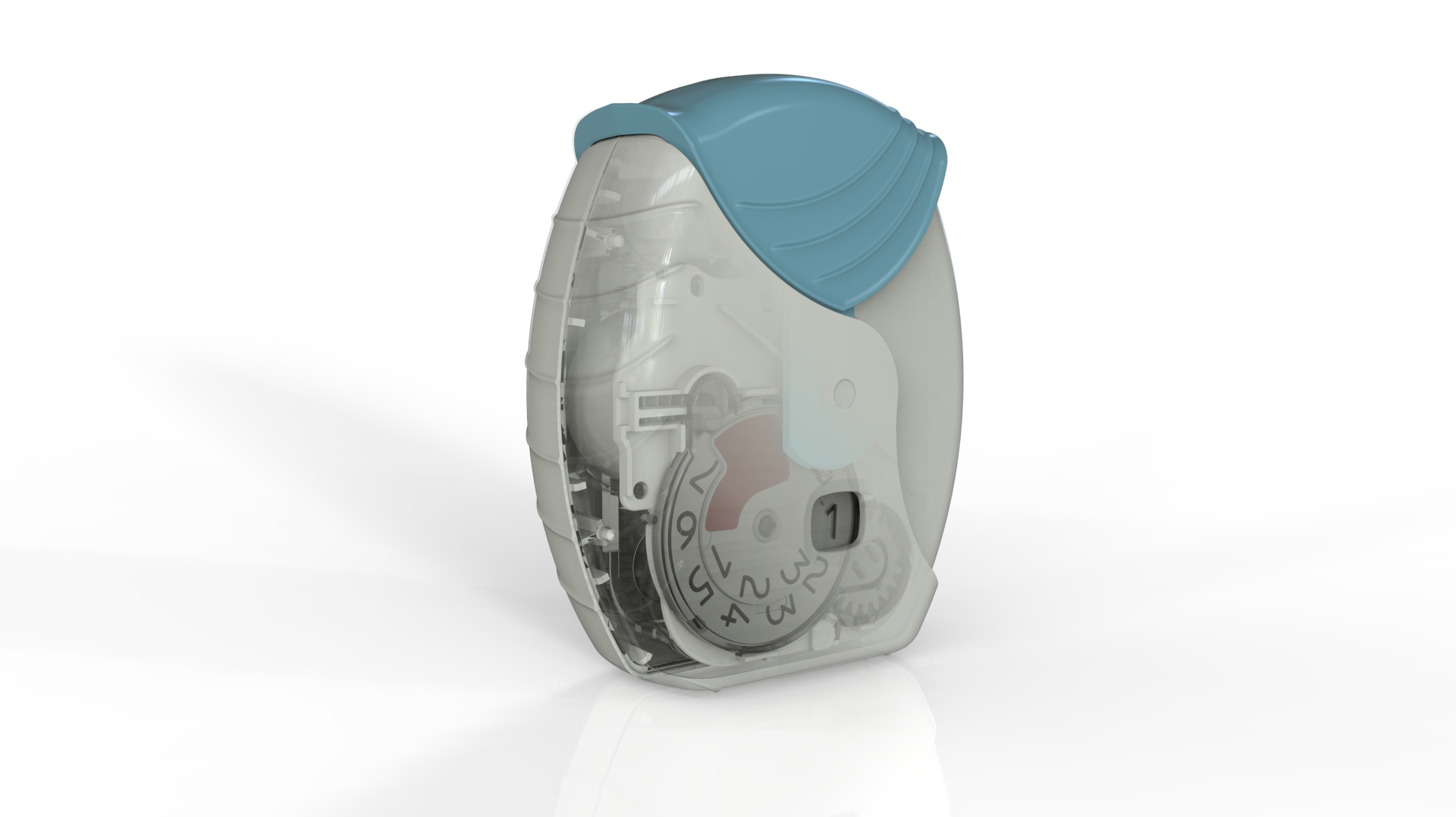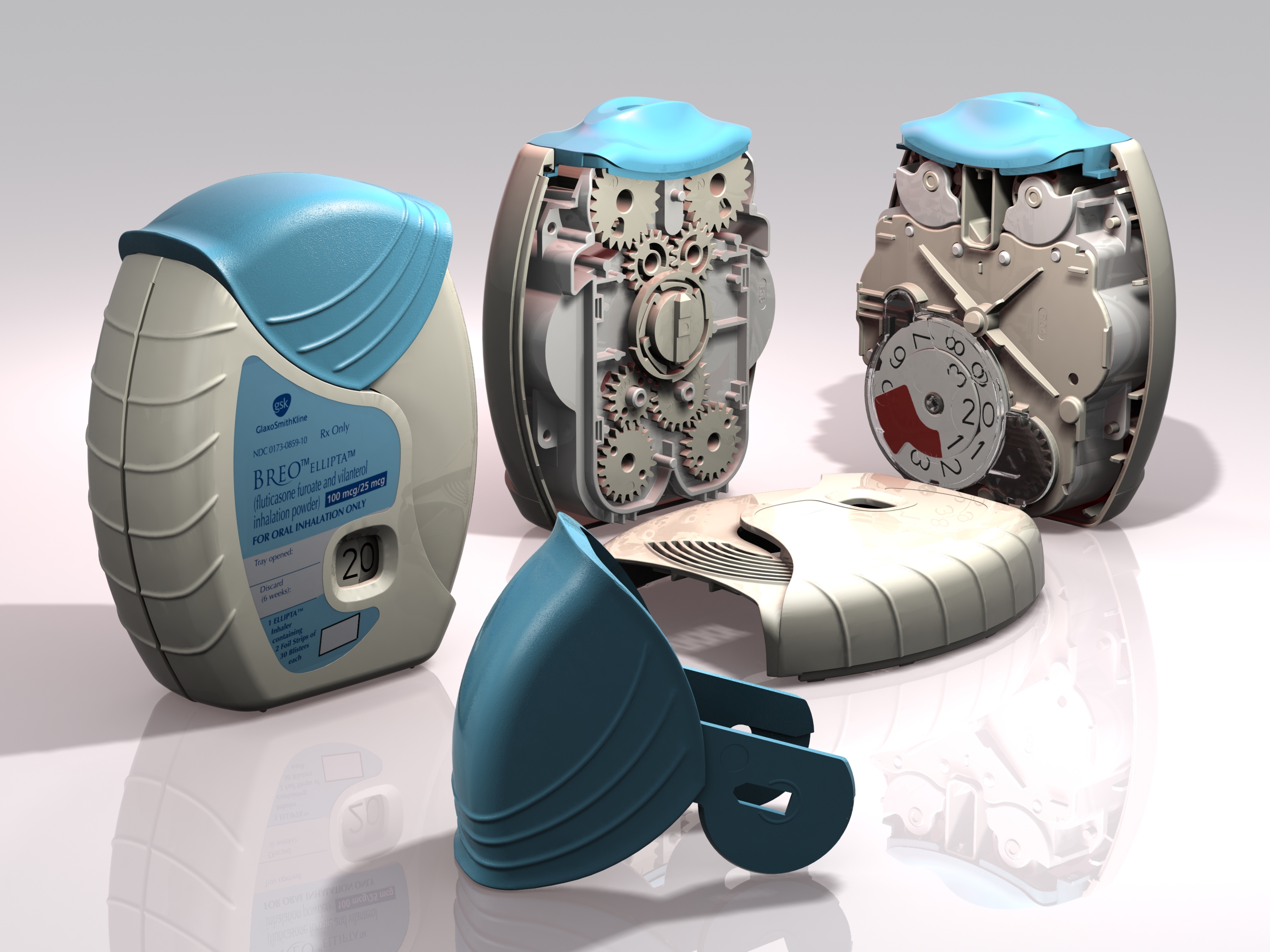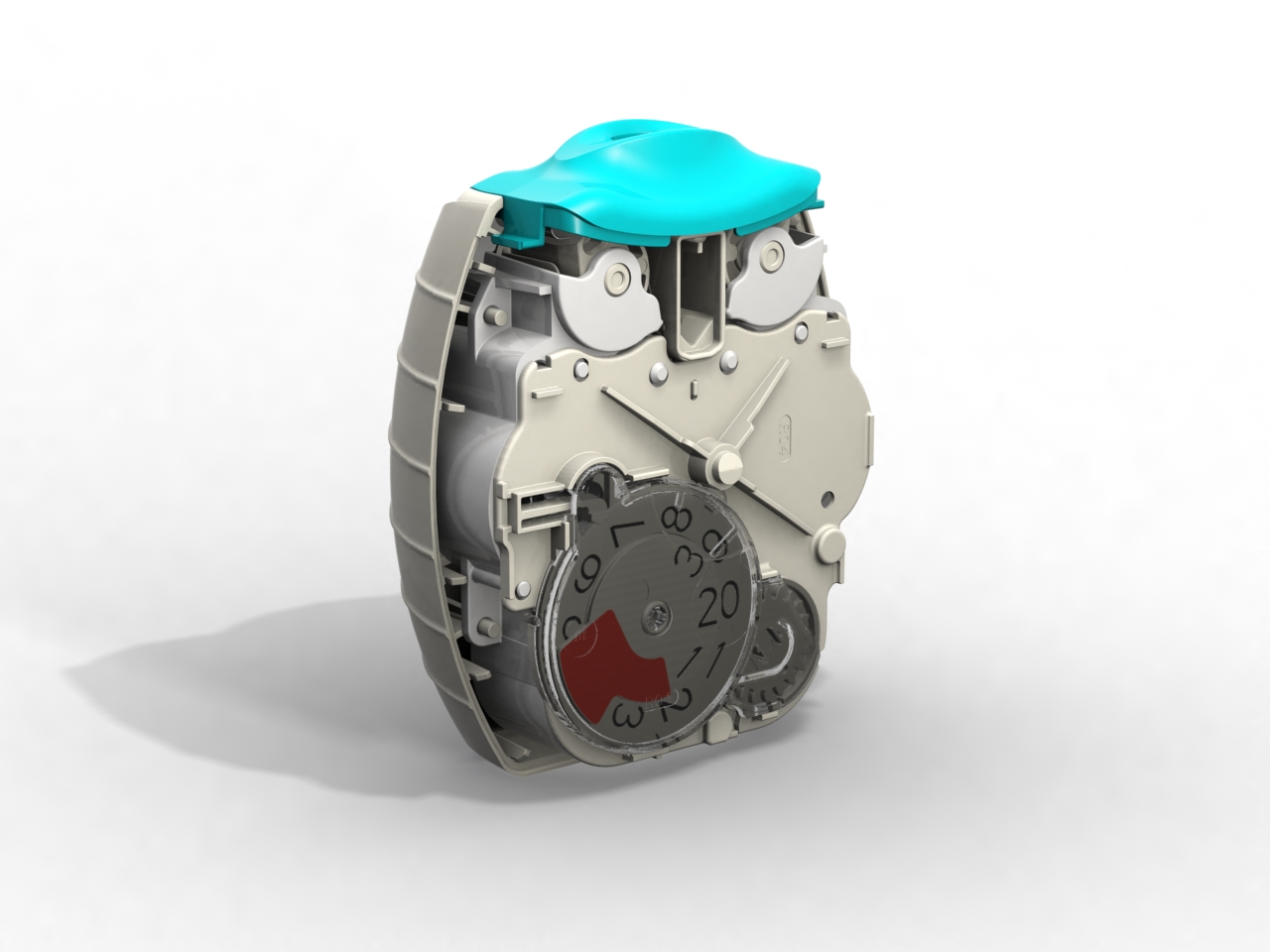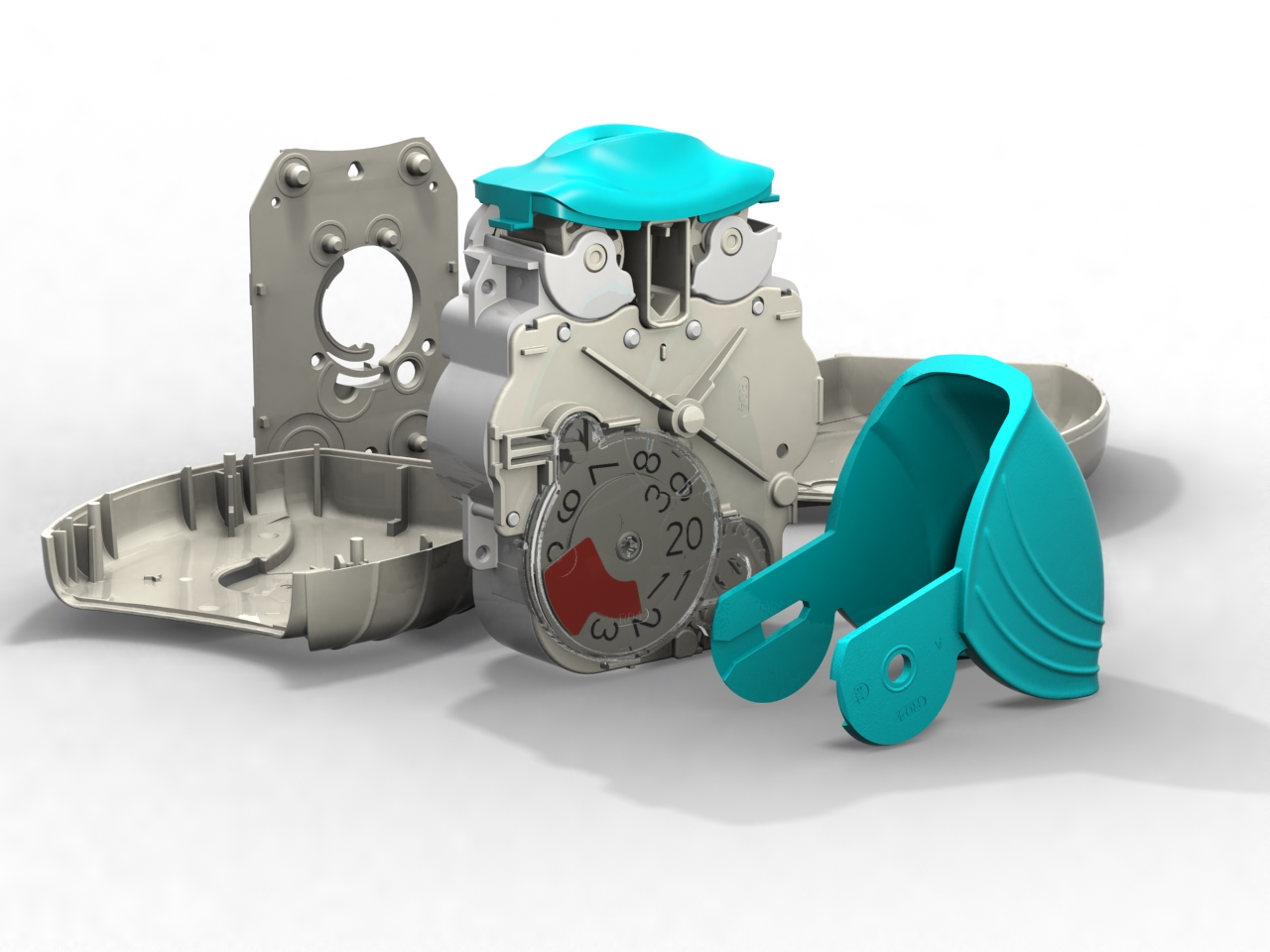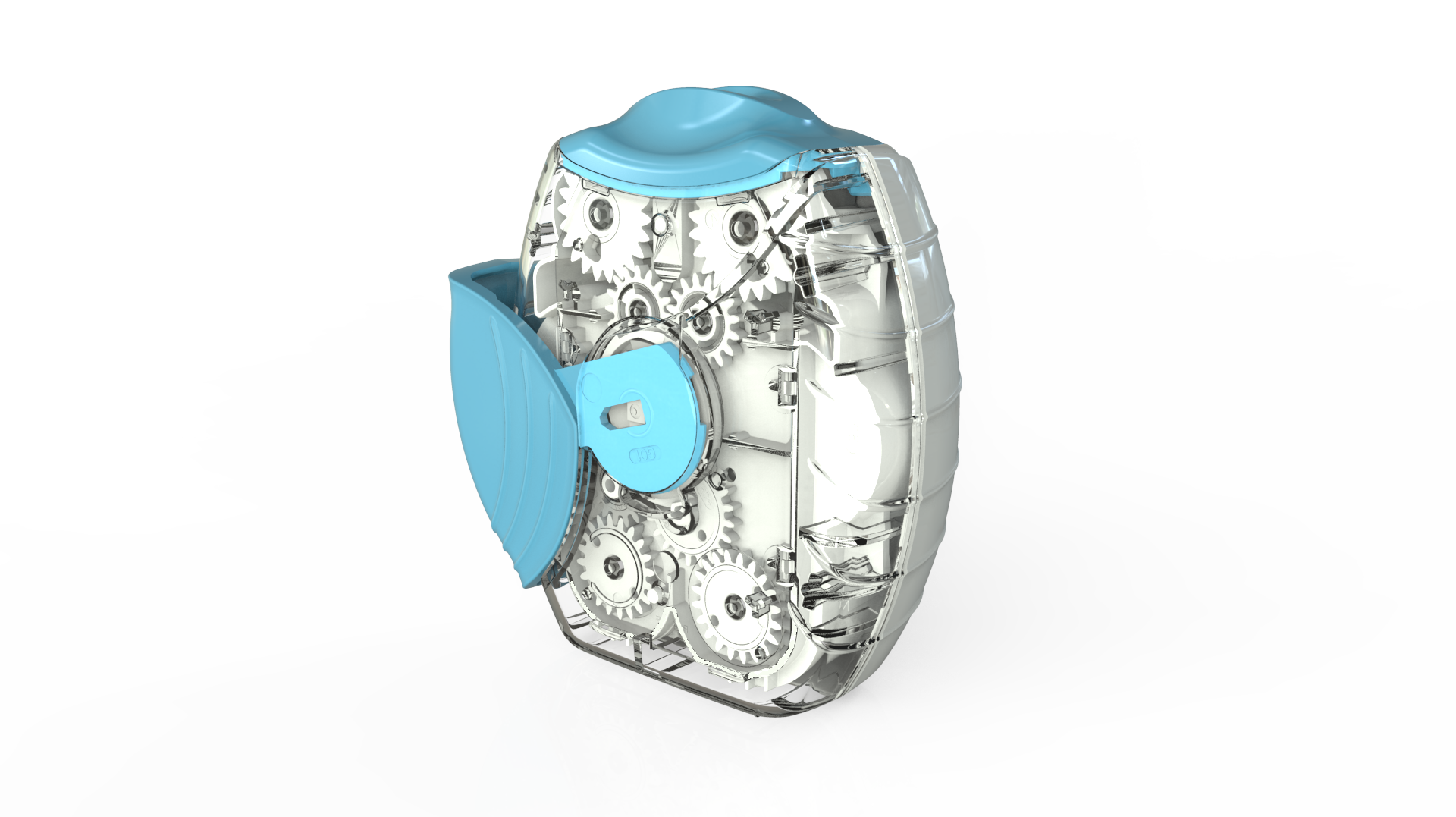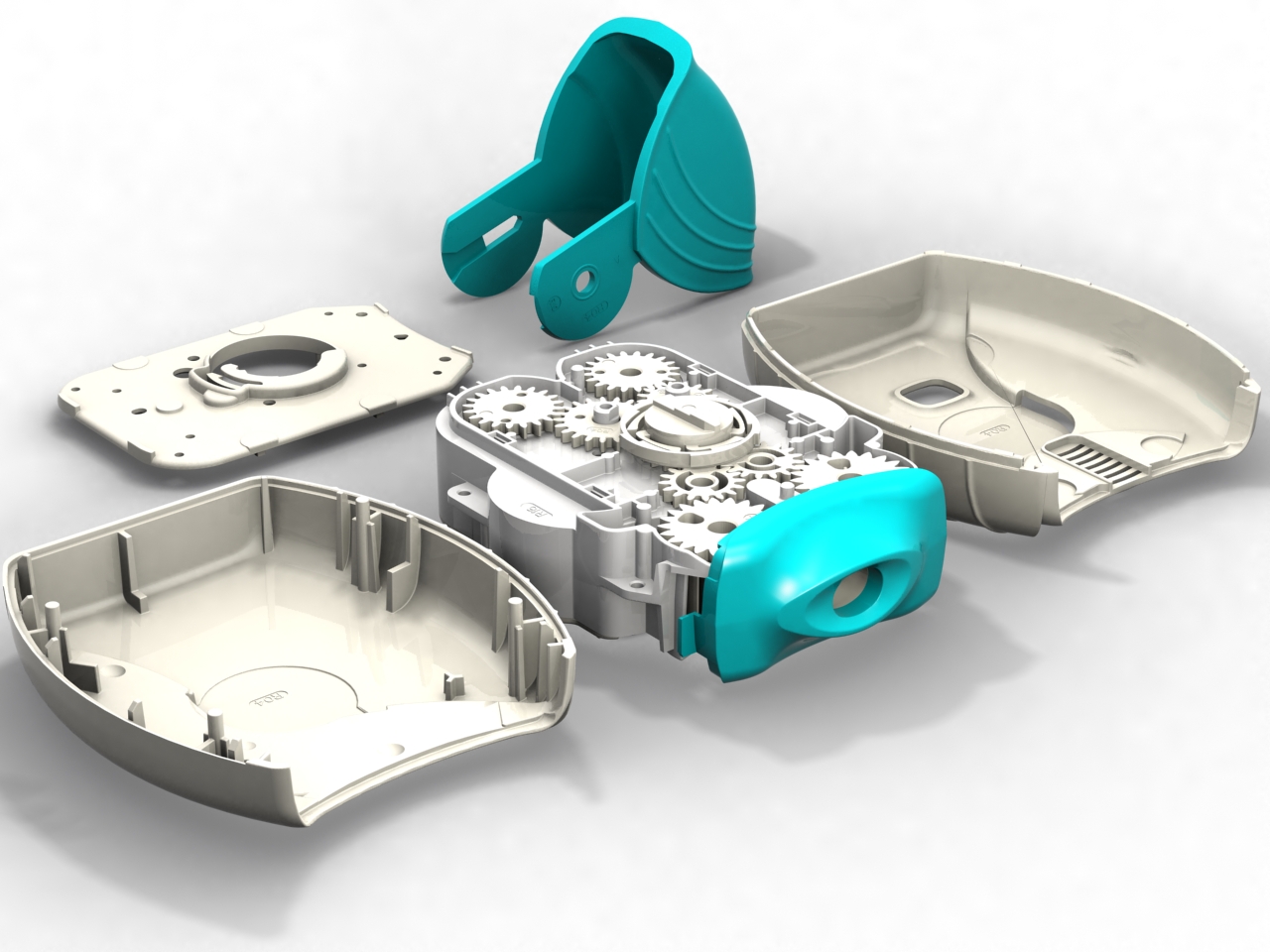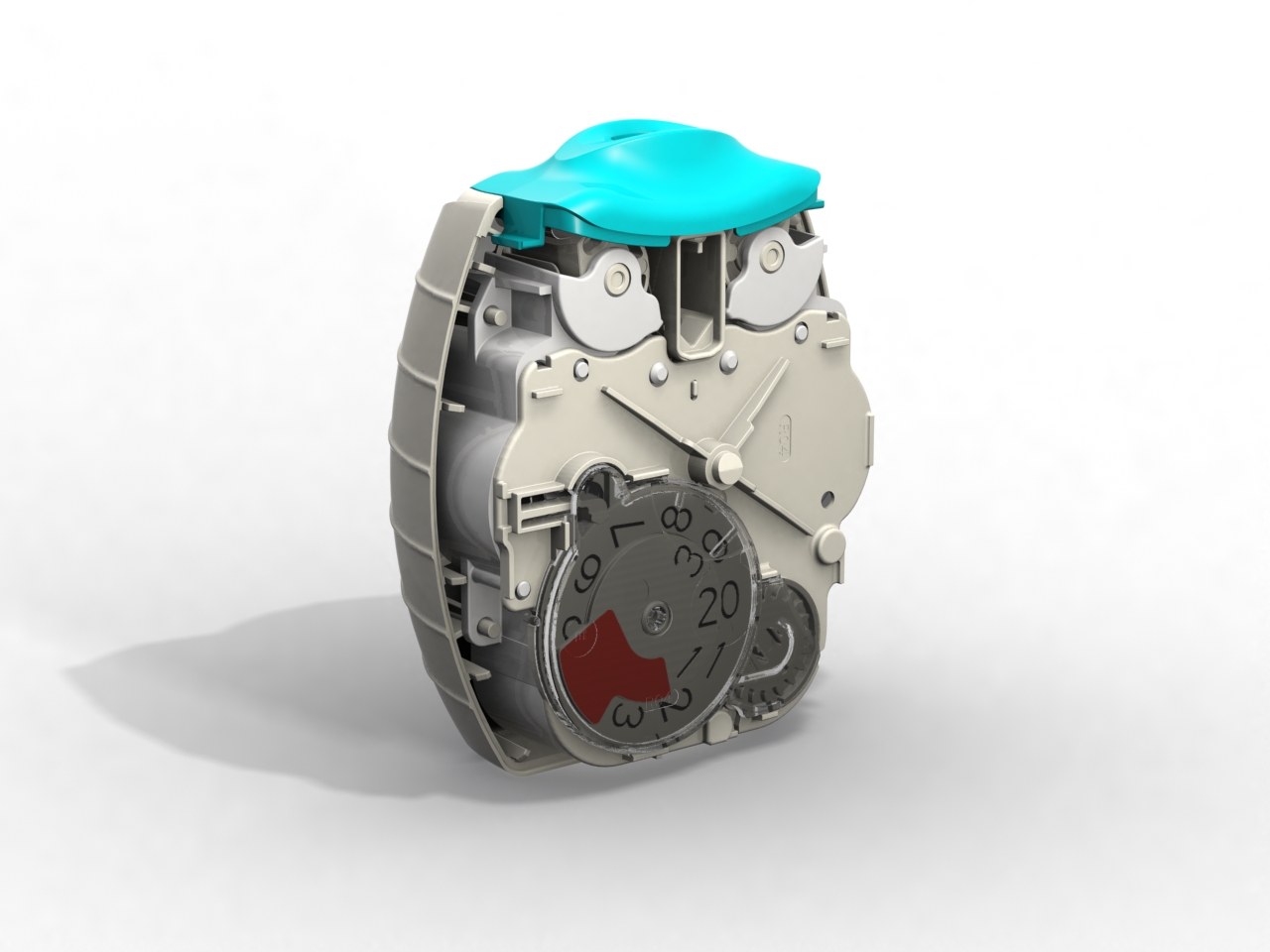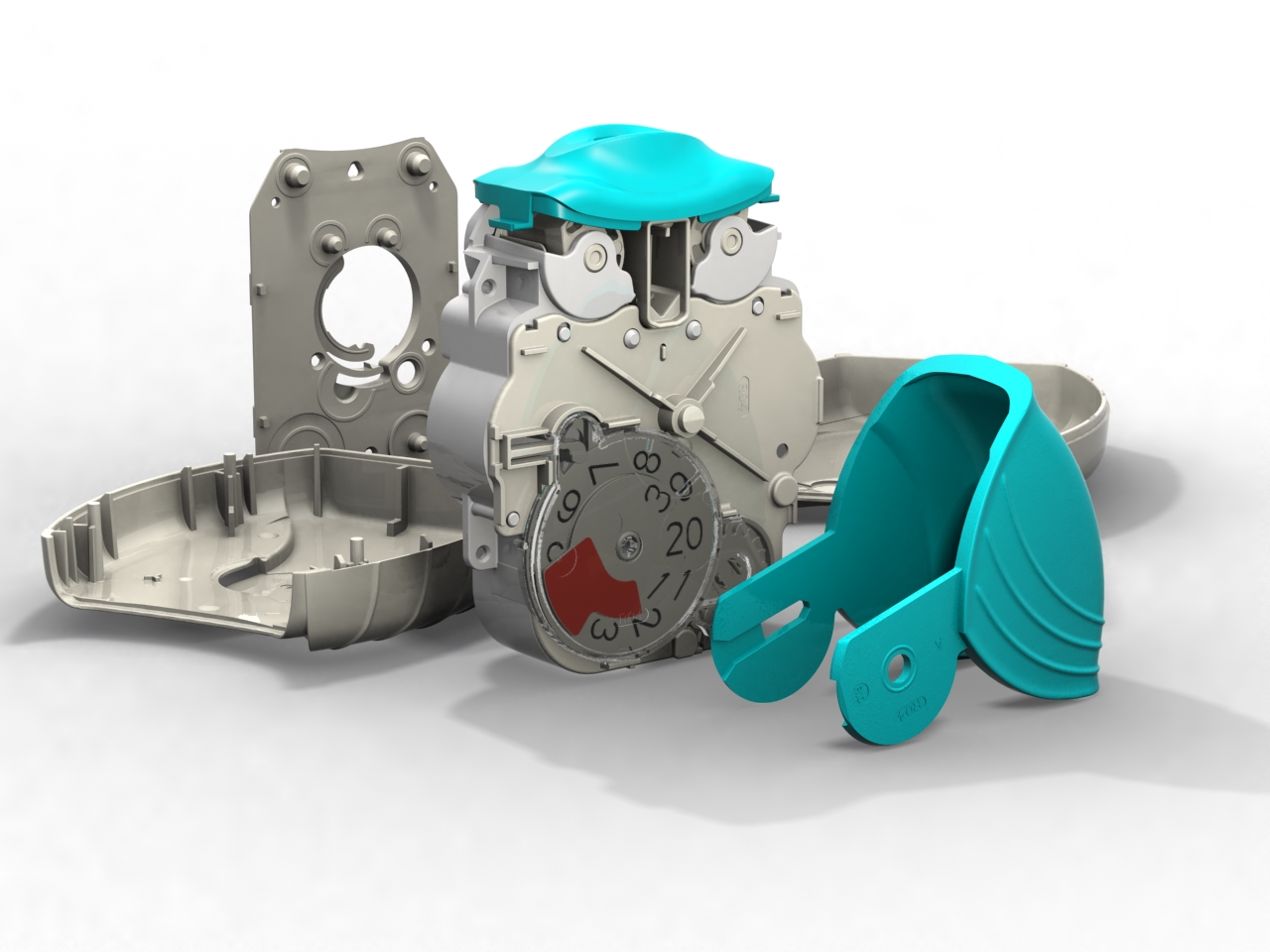
Following our work that created the Veramyst intranasal device and a relationship extending back eighteens year we were asked to develop the Ellipta twin strip inhaler.
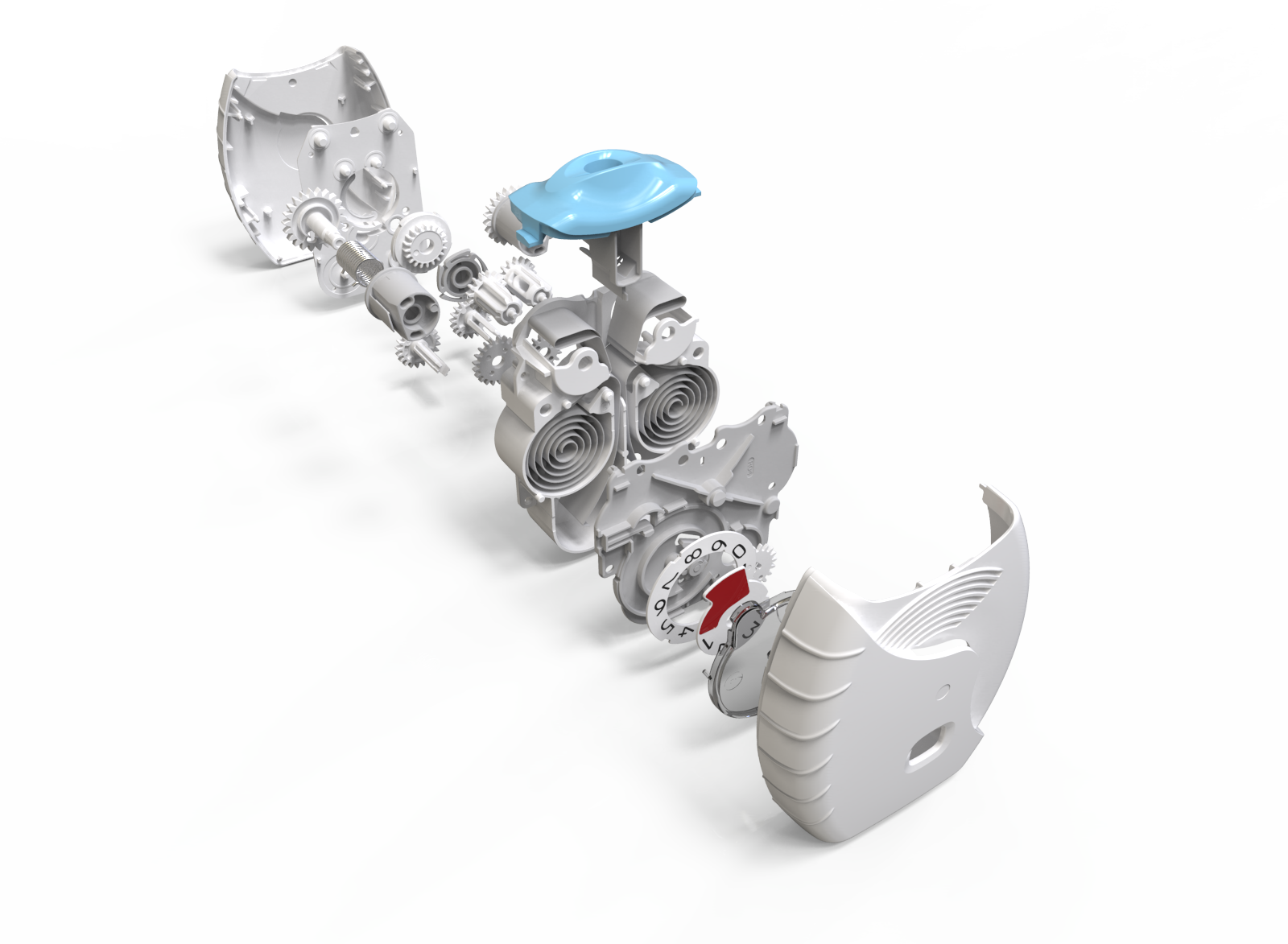
This complex and thoroughly resolved device represents eight years of development input undertaking:
Concept Layouts
Experimentation
Innovation
IP Generation
Detail Design
Prototype Parts
Test Machines
Design for Scale-up
Industrialisation
Toleranced drawing data
Support and Attendance for Usability Trials and FMEA
As a British design consultant we supply data directly to toolmakers and have very good relationships with high quality suppliers like Männer and Schotlii.
Specialised test and assembly equipment can be provided for some of the manufacturing operations. By Warwick Design providing these supplementary services in-house the client has benefitted from a quicker turnaround, with reduced knowledge transfer issues and number of external suppliers kept to a minimum.
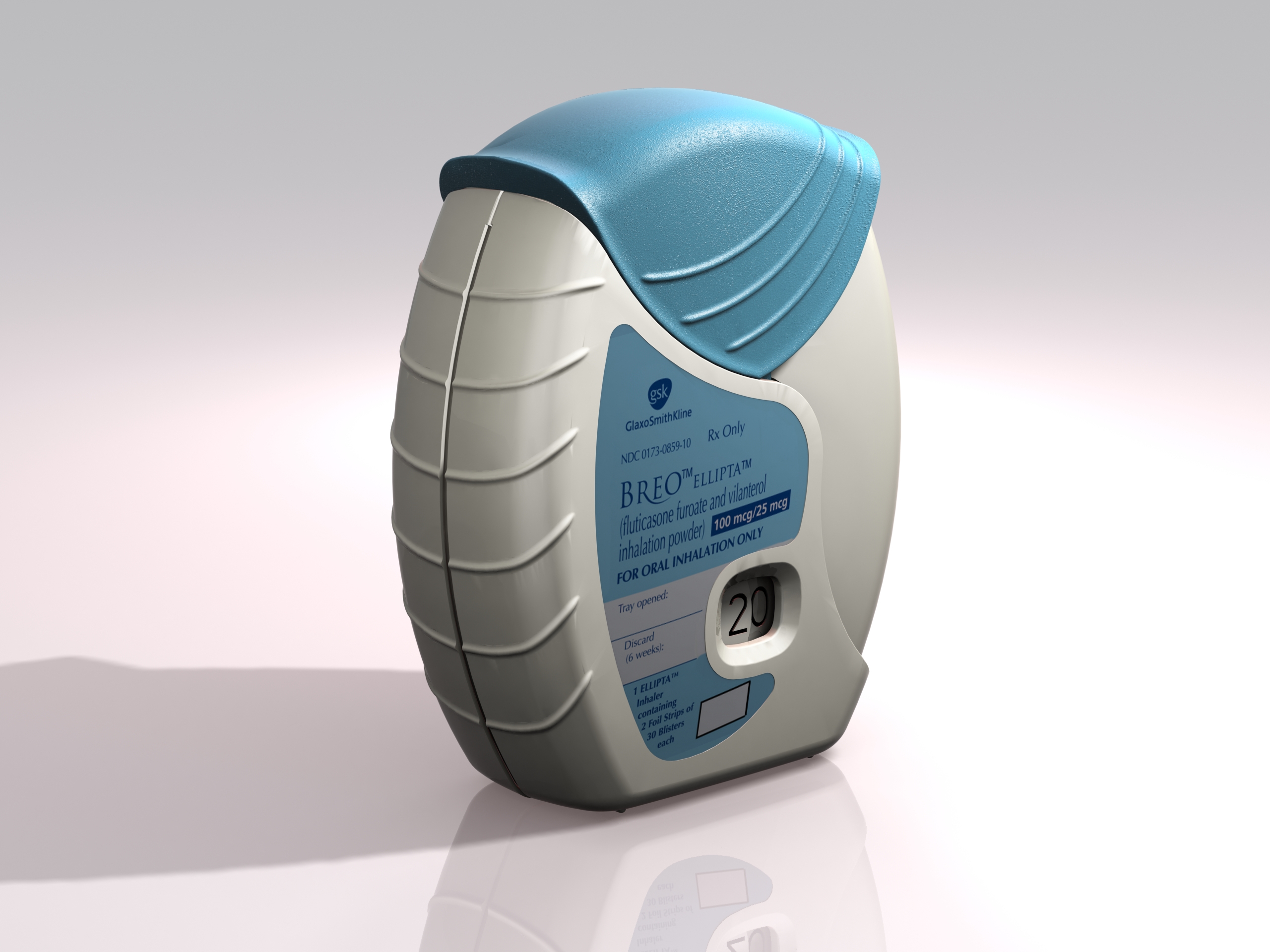
Conventional COPD treatment requires taking multiple doses from different inhalers. This new combination inhaler dispenses two active pharmaceutical ingredients that are inhaled simultaneously in a once-a-day regime supplied by the automatic opening of two individual blister strips.
Warwick Design looked at new ways of delivering two active pharmaceutical ingredients simultaneously. We produced a number of proof of principle working models that offered fantastic levels of control.
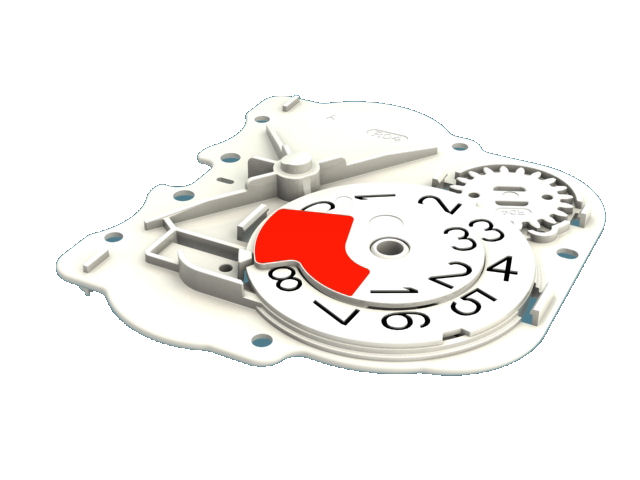
The device was conceived for straightforward open-inhale-close operation with a large character decrementing dose counter to provide the user with a clear indication of the remaining treatments.
Warwick Design has carried out extensive design, R&D and industrialisation developing a high level of confidence, ease of communication with an understanding of the partner’s rigorous requirements. It was designed to work consistently and reliably over a much wider dimensional tolerance band for the components than will ever be seen. The design therefore is extremely robust in use and the device has considerable manufacturing resilience.
Warwick Design created IP in the inhaler and was responsible for layout, the major portion of the design and detailing. Prototype steel tooling was manufactured from early development data enabling evaluation of the device’s performance and potential production requirements from first-off sample mouldings.
The refinement continued leading to Warwick Design providing component data for the clinical trial mouldings, industrialisation and scale-up stages. This was a highly collaborative project with weekly meetings at our office attended by all partners including Tech Group, Männer, Rexam and GSK’s own teams.
Ellipta has exceptionally reliable operation that required many years of development to perfect. The treatment is metered and dispensed by an intricate but robust device that is pharmaceutically reliable and particularly easy for the patient to use. Despite opening two strips the force to operate the lever is no greater than the single strip Diskus.
With so many moving parts extensive investigations were carried out in tolerancing, geartooth shaping and clearances at all extremes of moulding variations. This is to ensure that the device always delivers and always delivers both parts of the treatment.
Warwick Design also supported this device with assembly rigs that ensure pre-production quantities are put together correctly allowing the function of clips and welds to be assessed. Similarly we provided blister strip filling machines for laboratory scale manufacture.
A robust device, simple to use, reliable and easy to assemble by the million